Microlight cold laser ML830, technically termed as Low-level laser therapy (LLLT) is one of the smartest inventions of Microlight corporations. It is designed to treat the pain of muscles and joints, non-surgical disorders, neurological disorders, and connective and soft tissue pain through adjunctive therapy. The patented Microlight laser was the first low-level laser therapy that was approved by the FDA. After twelve years of research, the pioneer Microlight Company has received the U.S. FDA approval to sell their smart LLLT technology to provide temporary relief of musculoskeletal pain and other non-surgical disorders to assist patients with Carpal Tunnel Syndrome (CTS). ML830 is often referred to as a low energy laser (cold laser) with a wavelength of 830 nm per diodes. Read more about ML830, its working mechanism, and its uses.

In 1985, the renowned Microlight Company has developed the smart laser technology that is a low-level energy laser (cold laser) named Microlight cold laser ML830. In 1990, the smart laser was brought to the USA which was later approved by the FDA in 2002. It is the first 3B laser that is battery-operated, portable, and non-invasive that is designed to provide short-term relief of musculoskeletal pain, neurological disorders, and non-surgical disarrays. Unlike surgical and cosmetic lasers, ML830 is the world’s first hands free smart laser technology that does not damage body tissues by generating thermos-destructive heat and energy. The device is much more efficient and safe which has been used for over thirty years.

What is ML830?
The Microlight cold Laser ML830 is a manually handled, nonintrusive, light-radiating medical instrument operated by a battery. This device is recognized and authorized by the FDA for utilized in treating several disorders related to soft and connective tissue syndromes. It can also be used for the cure of certain neurological disarrays. The FDA has also permitted the sale of this device for the treatment of muscle problems. Microlight ML830 is a mild powerful laser device, hence simply termed as “cold laser”. The advantage of preferring the Low-Level Laser Therapy (LLLT) is that it is harmless to human tissues and can be used normally as compared to typical surgical and COSMETIC lasers that release heat in the form of thermo-destructive laser power and can cause several side effects. This is the reason, the FDA has Classified Microlight ML830 as a Class IIIB medical instrument.

The Microlight ML830 has been used in Low-Level Laser Therapy for the treatment of various medical problems such as soft tissue disorders for more than 30 years and has a successful history in medical research for health potency and safety. Being non-intrusive contact medical appliance, cold lasers are simple to use, portable, and provides trustworthy and persuasive treatment choices to the therapist and the patient.
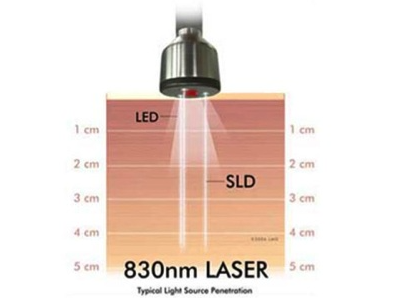
Working Mechanism of ML830
According to researches and experiments, it is proved that when light amenable chromophores in several tissues, in either cellular or sub-cellular regions, are illuminated with laser light, energy transmission takes place that stimulates more operative stages of working and improved relations. Laser light can penetrate deeper in comparison with other light wave types, initiates that improved synthesis of mitochondrial ATP, and generates expanded mast cell inflammatory extenuation, and encourages productive Ca2+ ion existence and production of useful and advantageous reactive oxygen species (ROS).
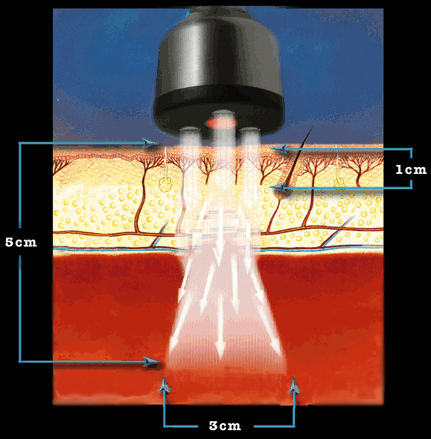
Due to the photo bio-stimulation process, oxidative metabolism is improved through cytochrome C oxidase and hemoglobin photosensitization. Excessive endorphin is released and improved prostaglandin synthesis takes place due to Laser illumination. Because of this influential impact photo-stimulation enzymes use, both ordinary and functional reactions take place that can cause inflammatory extenuation.
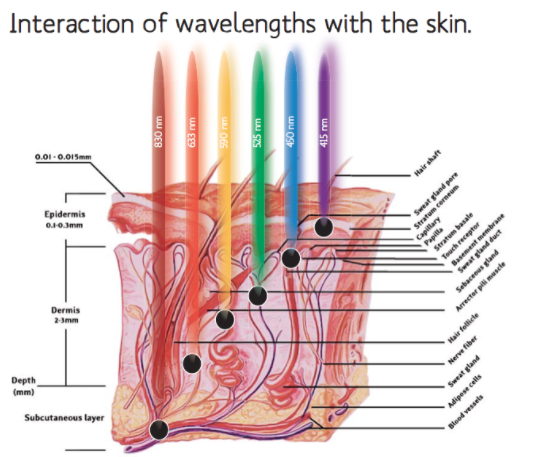
Why we use ML830?
ML830, the low-level laser therapy LLLT has the ability to provide a natural defense to the body and repair the injured body due to inflammation, injury, and other types of diseases. Research has shown that cold laser is also reliable in providing temporary relief of pain and to reduce injury and musculoskeletal disorder by altering the effects and controlling the extent of inflammation. It is also useful to repair the structure of tissues. The treatment is painless and can provide muscle tissue relaxation and temporary relief of other types of disorders. Some of the prominent results of LLLT are:
- Increased the richest protein in the human body named Collagen.
- Increased cell metabolism.
- Repaired injured bones and the structure of the tissues.
- Reduced the inflammation extent.
- Increased local blood circulation in the body.
- Improved nerve regeneration.
- Increased response of lymphatic and enzymes.
FDA CLEARANCE
The ML830 smart laser technology has been approved by the FDA, the USA in 2002. The chief objective of LLLT is to provide temporary relief of hand and wrist to the patient through adjunctive treatment with Carpal Tunnel Syndrome CTS. The device can provide relief of musculoskeletal pain, arthritis, and other non-surgical syndromes. Thus, promoting muscle tissue relaxation. This patented device can temporarily increase the local blood circulation in the body.
Conclusion
By summing up this article, it is concluded that ML830 is one of the smartest inventions of Microlight Company to provide temporary muscle and tissue relaxation and repair injured body components through adjunctive treatment with Carpal Tunnel Syndrome. This is a low-level laser therapy that produces infrared light of a wavelength of 830 nm per diodes. Clinical Research has proved that LLLT stimulates nerve function and increases cell metabolism. ML830 can provide defensive mechanisms in the body and repair injured body components by reducing the duration of inflammation. Thus, provide muscle tissue relaxation.

